Now Recruiting for Clinical Trial!
Need Help with Your Depression?


Trial Information

Individuals will receive treatment over a course of
6 weeks
About the Study
We are recruiting patients who suffer from Major Depressive Disorder (MDD) and have not responded to antidepressant therapies to participate in a clinical study. The study is designed to evaluate the effectiveness of a new, innovative and drug-free therapy using Brainsway’s Deep Transcranial Magnetic Stimulation (Deep TMS) device.
The goal of the study is to compare a new investigational stimulation protocol to the FDA-cleared standard-of-care stimulation protocol. All participants will receive active Deep TMS and participation is free of charge to all individuals.
Study Design
All participants will be randomized into one of two groups: a treatment group and a control group. Participants in both groups will receive TMS with the Brainsway Deep TMS device. The treatment group will receive the investigational stimulation protocol; the control group will receive the FDA-cleared standard-of-care deep TMS stimulation protocol.
The duration, frequency and treatment schedule will differ between the groups. Participants cannot choose if they are in the treatment or control group as group assignment is randomly generated.
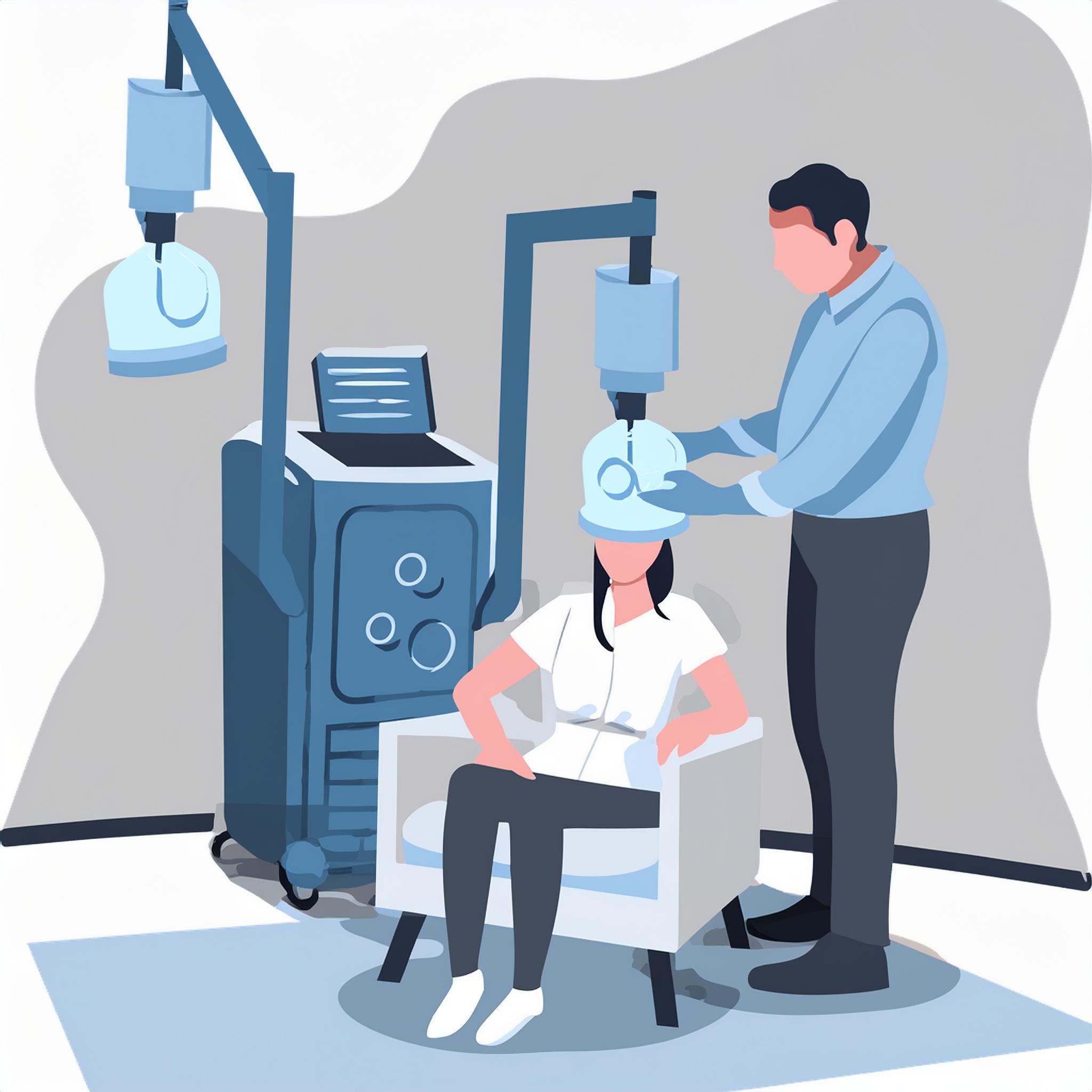
The patient is comfortably seated in a chair, and a cushioned helmet placed over the head generates brief magnetic fields.

Immediately after each session, patients can drive home independently and resume their daily routine.
Patients can continue their current behavioral or medication treatment during the study.
Eligibility Criteria
Study participants must :
Be between 22 and 68 years of age
Have a primary DSM-5 diagnosis of
Major Depression
Have a minimum of 1 and a maximum of 4 antidepressant trials in the current episode
Study participants must not :
Have any previous or current psychotic or bipolar disorder
Have an active alcohol or substance use disorder
Have a current diagnosis of post-traumatic stress disorder
Possess ferromagnetic metals implanted in the head or neck
Be pregnant or breastfeeding/chestfeeding
Have a personal or family history of seizures, not including febrile or ECT-induced seizures
To view additional study details including complete inclusion/exclusion criteria, please visit the National Library of Medicine at clinicaltrials.gov/study/NCT06357832
Trial Site: DTMS
Please make sure that you map out your proximity to the trial site:
1601 Forum Place, Suite 1005,
West Palm Beach, FL 33401
Meet Dr. Aron Tendler

Dr. Aron Tendler was born and primarily raised in NY. He graduated summa cum laude with a bachelor of arts from Yeshiva University and obtained his medical degree with distinction in research from SUNY Downstate in Brooklyn. Dr. Tendler trained in Internal medicine and psychiatry at Tulane University and completed his residency in Psychiatry at the University of Chicago in 2006.
He has owned a private practice in Palm Beach County continuously since 2006. Dr. Tendler is board certified by the American Board of Psychiatry and Neurology in general psychiatry and sleep medicine and by the American Board of Obesity Medicine in Obesity Medicine.
Dr. Tendler sees patients for twenty hours a week. Areas of strength for the practice are treating patients with treatment-resistant mood and anxiety disorders, autism, and schizophrenia.
Since 2015 Dr. Tendler has also been a vice president and chief medical officer for BrainsWay (TASE, NASDAQ). BrainsWay manufactures noninvasive brain stimulation systems with multiple FDA and CE clearances and over 950 locations worldwide.
About Deep TMS Technology

Brainsway Deep TMS is an innovative, revolutionary, non-invasive technology for the treatment of Major Depressive Disorder.
Deep TMS non-invasively stimulates the brain regions responsible for depression using brief magnetic fields, at an amplitude similar to that used in magnetic resonance imaging (MRI) systems. The device has been cleared by the FDA for treating depression, obsessive compulsive disorder, and smoking addiction and has been studied in over 100 clinical trials around the world.
Deep TMS treatment involves visits to a clinic multiple times per week for several weeks. Each Deep TMS session lasts 20 minutes or less. The side effects are generally mild and can include headache or scalp discomfort at the treatment site.
Since it does not involve medication, there are no systemic side effects, which is appealing for individuals who cannot tolerate drugs.